Chromium in the Garden
go.ncsu.edu/readext?826036
en Español / em Português
El inglés es el idioma de control de esta página. En la medida en que haya algún conflicto entre la traducción al inglés y la traducción, el inglés prevalece.
Al hacer clic en el enlace de traducción se activa un servicio de traducción gratuito para convertir la página al español. Al igual que con cualquier traducción por Internet, la conversión no es sensible al contexto y puede que no traduzca el texto en su significado original. NC State Extension no garantiza la exactitud del texto traducido. Por favor, tenga en cuenta que algunas aplicaciones y/o servicios pueden no funcionar como se espera cuando se traducen.
Português
Inglês é o idioma de controle desta página. Na medida que haja algum conflito entre o texto original em Inglês e a tradução, o Inglês prevalece.
Ao clicar no link de tradução, um serviço gratuito de tradução será ativado para converter a página para o Português. Como em qualquer tradução pela internet, a conversão não é sensivel ao contexto e pode não ocorrer a tradução para o significado orginal. O serviço de Extensão da Carolina do Norte (NC State Extension) não garante a exatidão do texto traduzido. Por favor, observe que algumas funções ou serviços podem não funcionar como esperado após a tradução.
English
English is the controlling language of this page. To the extent there is any conflict between the English text and the translation, English controls.
Clicking on the translation link activates a free translation service to convert the page to Spanish. As with any Internet translation, the conversion is not context-sensitive and may not translate the text to its original meaning. NC State Extension does not guarantee the accuracy of the translated text. Please note that some applications and/or services may not function as expected when translated.
Collapse ▲Sources of Chromium Exposure
Chromium is not easily taken up by plants, so direct contact with contaminated soil is the most common exposure pathway in the garden. This can include breathing in or eating soil particles directly or from soil covered-produce, tracking soil inside the home, and breathing or eating the soil at a later time. Chromium exposure can occur in and outside the garden.
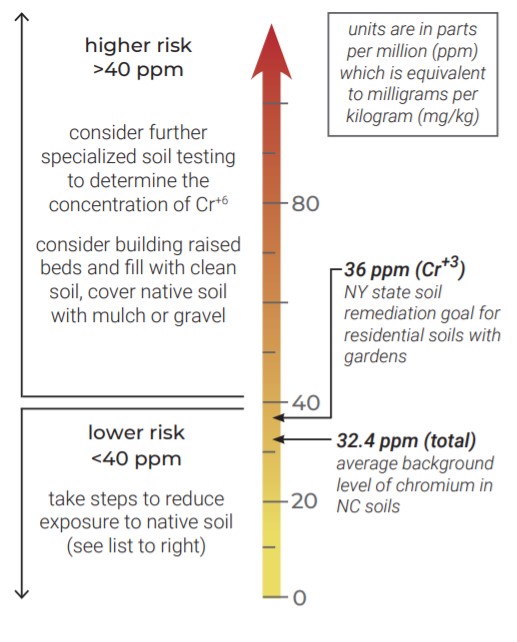
Making Sense of Regulatory Standards
No official standards have been established in North Carolina for acceptable levels of chromium in garden soils. For remediating soil at industrial sites, North Carolina uses EPA’s guideline of 24,000 ppm for Cr+3, and0.3 ppm for Cr+6. The guidelines below can help you contextualize the chromium levels in your garden soil.
Health Impacts of Chromium
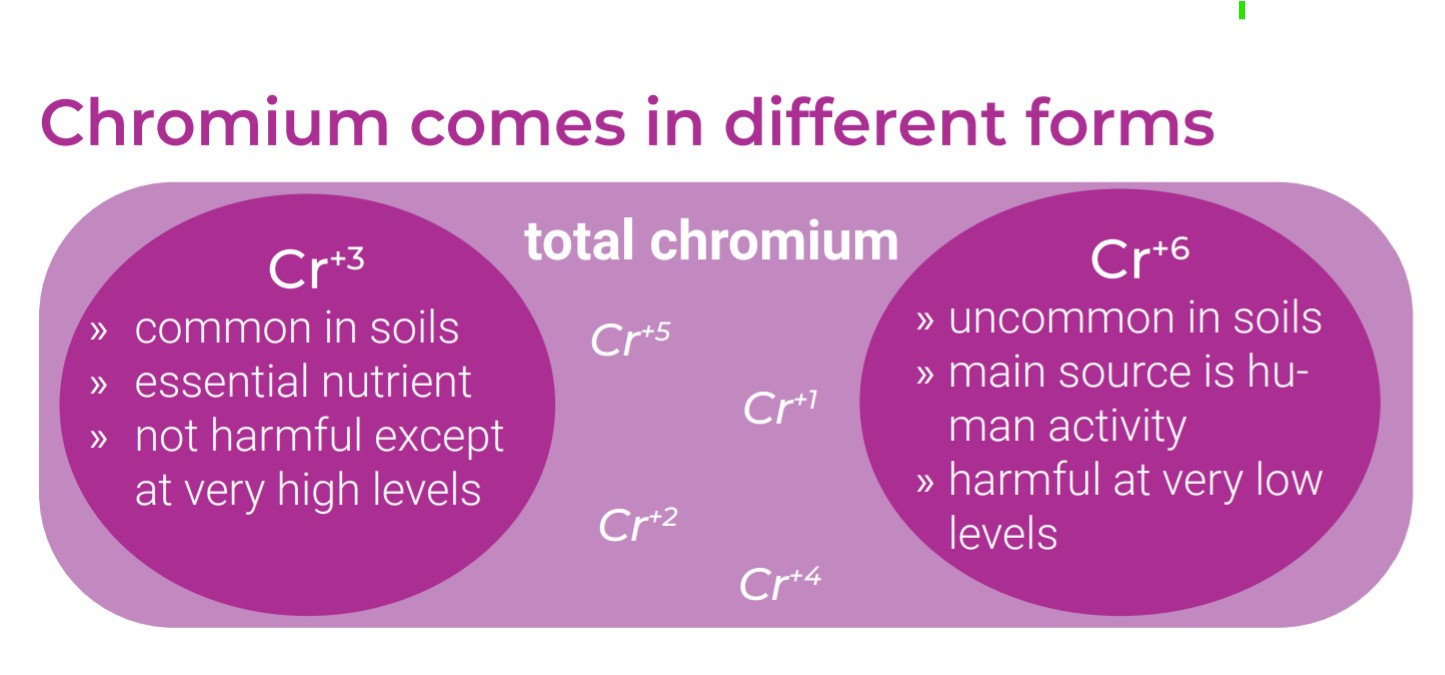
Cr+3, the more common form, is a beneficial nutrient in small doses and is not easily absorbed into the body. Cr+6, however, is a known carcinogen when inhaled and therefore has no “safe” level of exposure.
Exposure to Cr+6 can increase your risk of developing breathing difficulty, asthma or allergy-like symptoms, stomach ulcers or irritation, anemia, lung cancer, and more. Long-term exposure to Cr+6 may affect the male reproductive system.
It is unknown whether children are more vulnerable to the effects of Cr+6 specifically, but it is still important to limit their contact with chromium.
Reduce Chromium Exposure in the Garden
- Adding compost or other organic matter from a contaminant-free source may help convert Cr+6 into Cr+3. Check the NC Composting Council website to find STA or OMRI certified compost
- Thoroughly wash produce grown in chromium-contaminated soil
- Avoid growing root vegetables in soils with high levels of chromium, and peel root vegetables before eating to further minimize risk
- If applicable, test well water sources for chromium, specifically Cr+6
- Conduct a soil safety training to teach exposure reduction strategies to all garden users
- Visit our website below for our factsheet on 10 Healthy Garden Habits
Testing Resources
 |
Well water testing for chromium |
 |
How to test your soil and interpret the results |
 |
Email questions about chromium soil testing |
Email us at superfund@duke.edu
This factsheet was created by the Duke University Superfund Research Center’s Community Engagement Core with the goal of helping garden managers, Extension agents, Master Gardeners, and home gardeners identify, understand, and manage risks associated with chemical contamination that may be present in garden soils.
This work was supported through the National Institute of Environmental Health Sciences P42 Multiproject Center Grant program, grant number P42ES010356.